Death rays and thermal radiation
Posted by David Zaslavsky on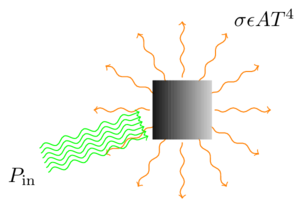
It’s been far too long since I did a Mythbusters writeup, but I think it’s time to stop stalling and bring this series back. On this week’s episode, Adam and Jamie tested the myth of Archimedes’ heat ray for a third time — that has to be some kind of record — at the request of President Obama.
The gist of the myth is this: by focusing enough of the sun’s rays, using a large number of mirrors, on an enemy ship, the Greeks hoped to heat it up enough to make it catch on fire. So far (spoiler alert), there’s no evidence that this thing ever could have worked. All three of the Mythbusters’ tests have failed.
But I think I can shed some light (no pun intended) on why. As it happens, I taught a lab on thermal radiation transfer this week, and that (along with an interesting perspective on gravitationally baking a turkey) reminded me that it’s fairly straightforward to calculate, at least in a simple model, the amount of radiation it takes to heat something up to a particular temperature. It all stems from the Stefan-Boltzmann equation,